CHEN-7250 Homework
Assignment No. 1
Due: 01/22, Fri
1. A tank
contains water solution of A. The initial concentration of A in the tank is 10
g/L. At time = 0, additional A is fed into the tank at the rate of 1 L/minute.
The concentration of A in the input stream is 20 g/L. At the same time the
liquid in the tank is withdrawn through a pipe connected at the bottom of the
tank. The flow rate through this pipe is v = (0.005)V,
L/minute, where V is the volume of liquid in the tank. The liquid is well
agitated. The density of liquid stays constant. A chemical reaction of A→B
takes place in the tank: rA =
- (0.08) CA g/L.min.
a. Using Polymath, determine the amount of A, the
concentration of A, and volume of liquid in the tank when time = 50 minutes.
b. Repeat part (a) by hand calculation. Hint: Take
NA (not CA ) as
the dependent variable.
c. Calculate the conversion (X) in this reactor
(considering the meaning of conversion) by Polymath when time = 50.
d. If this reactor is operated for a long period of
time, it will eventually reach a steady-state turning into a CSTR. Calculate
the conversion for this CSTR,
(i)
By hand
calculation using mole balance equation
(ii)
By
Polymath (taking a large number of t)
Assignment No. 2
Due: 01/27, Wed
Problem 3-17
Additional Question: (c) Calculate the reaction
time to achieve 50% conversion. Use polymath. Change k1 value to
1000.
Assignment No. 3
Due: 01/29, Fri
1. Starting from mole
balance equation of PFR, derive Eq. 4-17, p.170 for a second order isothermal
gas reaction. Show the derivation process. Picking up the equation from the
Appendix in not acceptable.
2. Problem 4-12 (a)
Assignment No. 4
Due: 02/01, Mon
1. Problem 4-7 (a), (b), (f)
with a revision: redo (a) and (b) only.
2. Problem 4-13
Assignment No. 5
Due: 02/03, Wed
1.
Problem
4-7 (e) and (f)-batch reactor. Ignore cost. Assume down-time for batch cycle
(empting, cleaning, refill, etc.) = 4.5 hours.
2.
Hint: Prove in terms of Damkohler
number.
Assignment No. 6
Due: 02/05, Fri
1.
Problem 4-23 (a),
(c).
Notes:
Set t(f) = 15,000.
Part (a): Conversion is
based on ethylene chlorohydrin. Note unit of k is in hour, not minute.
Part (c): Consider using
if( ) then( ) else( ) statement in Polymath. See
Polymath examples
2.
(Unsteady-State
CSTR)
An elementary gas reaction, A —> 2B, is taking
place in an unsteady-state CSTR. The reactor is initially filled with an inert
gas. At time zero feed containing A only is fed into
the reactor.
(a)
Plot CA,
CB, and CI as a function of time
(b)
How long would it
take for CB to reach 50% of CA0?
(c)
Calculate the
conversion at this point and the steady-state conversion.
Additional information:
-rA = 0.1 CA mols A/(min,)(L)
Pressure is uniform at 1 atm
throughout, T = 300°K, vo =
1 L/min., V = 40 L
Notes: Consider using total mole balance
and that NT = NA + NB +
NI = constant in the reactor (IG Law).
Assignment No. 7
Due: 02/08, Mon
Problem 4-17 (a), (b)
Note: This is a trial and error problem with two
unknown parameters (α and kCA02/FAO).
Assignment No. 8
Due: 02/10, Wed
The
following elementary liquid reactions are carried out by two different methods
as described below.
Using Polymath calculate the yield and the selectivity (SDU) for
each case.
A+B→D, k1 = 0.25 (mol/L)-1(minute)-1
A+2B→U, k2A = 0.66 (mol/L)-2(minute)-1
(Method 1)
Reactants A and B are kept in two separate vessels (Tank A
and Tank B). The volume of
each reactant is 50 liters. The concentration of reactant A is 0.8 mol/L and that of reactant B is 1.2 mol/L. The
reactant B is poured abruptly into Tank A, mixed well, and the reaction is carried out in
batch mode for 60 minutes.
(Method 2)
Reactant B is gradually added into
Tank A at a constant flow rate of v0 until Tank B is emptied (semi-batch operation).
Assume the reaction stops at this point. The flow rate, v0, is
adjusted such that the
final conversion of A is equal to that of Method 1. Note that t(f) varies depending on v0.
Assignment No. 9
Due: 02/12, Fri
1. For
an elementary reaction of A→ B→ C,
(a) Determine CB
and tmax (for CB) in a batch reactor.
(b) Determine CA, CB, Cc, YB, and SBC in a CSTR as function of t and ki.
2. Problem 6-10 (a), (b), (c)
Additional
question in part (b): Make plots of CB, YB, SBC, and
conversion vs. temperature. Note that one can make a plot of any given function
using Polymath by putting in a dummy differential equation in the DE section
and list a given function in the EE (explicit equation) section.
Assignment No. 10
Due: 02/19, Fri
1. The following parallel
reactions are taking place in a batch reactor for the duration of 30 minutes.
1
A
→ B
2
A
→ C
Data:
CA0
=1.5 mol/L
k1
= 0.012, k2 = 0.002 min-1 at 300oK
E1
= 6000 cal/mol, E2
= 11000 cal/mol
(a)
The
reactor runs isothermally. Find the reaction temperature that will maximize
production of B (CB).
(b)
The
reactor is now to be operated non-isothermally. We will consider applying a
temperature profile that changes linearly with time such that T = a + bt, where a and b are constants
and t is reaction time. Find a and b that will
maximize the production of B. Explain the difference in answers between part
(a) and (b).
2. Problem 6-14 (a), (b), (c) (20-points)
Assignment No. 11
Due: 02/22, Mon
Problem 8-6 (a) – (d)
Assignment
No. 12
Due: 02/24, Wed
1. Problem 8-9 (a) – (d) with the following revisions.
Change Ua/ρb to 3.0 (from 0.08) in (a)
Change α value to 0.0075 in (a-3)
Repeat (a-1) only in (b) and (d)
2. Problem 8-18
Ignore
all the questions of (a)-(j).
Calculate
the reactor temperature and conversion. There are more than one solutions. List
all the solutions you can find.
Assignment
No. 13
Due: 02/26, Fri
Problem 8-12 (a) – (d)
Assignment
No. 14
Due: 03/04, Fri
Problem 8-18 (a)-(d),
(f), (g)
Question (b) modified:
What are the ignition, and extinction temperatures?
Assignment
No. 15
Due: 03/09, Wed
1.
The
following liquid parallel reaction takes place in a PFR.
A → B, k1
= 3.2x10-4 (minute)-1 at 300°K
A → C, k2 = 1.2x10-5 (minute)-1 at
300°K
E1 = 18,000 cal/mol
E2 = 24,000 cal/mol
vo = 2.0 L/min
V= 10L
CAO = 1.0 mol/L
CpA = CpB = Cpc =18.0 cal/mol
Cp coolant = 1 cal/g
HA = 5,000 cal/mol
HB = 1,600 cal/mol
HC = 4,000 cal/mol
Ua = 4.0 cal/min.°K.L
To = Feed temperature = 350°K
Tao = Inlet coolant temperature =310°K
(a) The PFR is a shell-and-tube
type with co-current cooling. Determine the flow rate of coolant (mc, g/minute)
that will maximize production of B. Calculate the yield of B and Selectivity
(B/C) and conversion of A under this condition.
(b) Determine the mcopt for maximum production of B under the
constraint that
YB/A > 0.8
2.
(20-points
max.)
The following multiple gas
reaction takes place in a CSTR.
1 2
A →B →C
3↓
2D
Data:
The reaction is elementary.
Feed:
Pure A, PAO = 4 atm, vo = 5 L/minute, To = 300°K
Reactor volume = 100 L
k1 = 1.5x10-2, k2 = 8.9x10-4, k3B = 2.5x10-4 minute-1 at 300°K
E1 = 5600, E2 =
6000, E3 = 7000 cal/mol
(a)
Find the optimum reactor
temperature that will maximize production of B.
(b)
Repeat (a ) with a constraint
that SCD >1.5
(c)
We now have the following additional information.
CPA
= CPB = CPC = 16.0 cal/mol.°K
CPD
= 10.0 cal/mol.°K
HA°
= 14600 cal mol, HB°
= 5500 cal mol, at 298°K
Hc°
= 4000 cal mol, HD°
= 3000 cal mol, at 298°K
Note that ∆Cp ≠ 0 for reaction No.3.
The reactor runs at 400°K.
Calculate Q (heating or cooling rate).
Assignment
No. 16
Due: 03/11, Fri
Problem 9-5 with the following revision.
kr = 0.002 min-1
Assignment No. 17
Due: 3/21, Mon
Problem 5-6. (a), (b). Use
differential and integral linear methods in (a).
Assignment No. 18
Due: 03/23, Wed
1. Problem 5-9.
Try both differential linear and integral linear
methods in part (a).
2. Problem in CD: P5-GB.
The oxidation of propene (P) to acrolein (A) was carried out over a Mo-Pr-Bi catalyst [Ind. Eng. Chem. Res., 26, 1419
(1987)].
CH3CH=CH2+O2![]() CH2=CHCHO+H2O
CH2=CHCHO+H2O
It has been proposed to correlate the data using
the power law model
for the rate law [cf. Equation (5-2)].
racrolin = k PpαP02β
The reaction was carried out in a differential reactor
with 0.5 g of catalyst at 623 K. From the data below, determine the reaction
orders with respect to propene, α, and oxygen, β, and the
specific reaction rate, k.
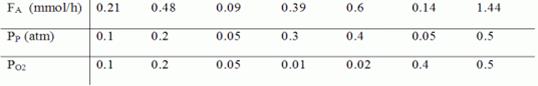
where
FA=exiting molar flow rate of acrolein, mmol/h
PP= entering partial pressure of propene, atm
PO2= entering partial pressure of oxygen, atm
Solve by differential linear and nonlinear
method.
Assignment No. 19
Due: 03/25, Fri
Problem 7-11 (a), (b). Consult Eq. 7-26
Assignment No. 20
Due: 03/28, Mon
Problem 5-5 (a), (b).
Assignment No. 21
Due: 04/04, Mon
1. Show that ![]()
where ![]() and L = half the slab
thickness
and L = half the slab
thickness
2. Problem 12-6 (a), (b)
Assignment No. 22
Due: 04/06, Wed
1. Using Polymath, calculate the
effectiveness factor for a flat-plate catalyst carrying out a second-order
reaction,
where, ![]() = 3.0
= 3.0
2. Derive a differential
equation describing CA vs. r in a long cylindrical catalyst, wherein
a first-order reaction is taking place.
a. Put the DE and the
boundary conditions into dimensionless form.
b. Calculate the effectiveness
factor using Polymath and by the analytical solution (Bessel function
solution), where ![]() = 8.0
= 8.0
c. Estimate an approximate
effectiveness factor using the flat-plate solution and appropriate![]() value (a universal Thiele modulus).
value (a universal Thiele modulus).
Assignment No. 23
Due: 04/08, Fri
A catalytic reaction experiment was carried out using a differential
reactor packed with spherical catalyst particles. The catalysts were then taken
out of the reactor, cut into two pieces (hemi-sphere form), reloaded, and the
same experiment was repeated. The data indicate the reaction rate increased by
20% in the second experiment. Estimate the effectiveness factor and the Thiele
modulus for the spherical catalyst. Assume first-order reaction.
Assignment No. 24
Due: 04/09, Mon
1. (5 points): From the class notes of
April 8 (the last Equation of Bischoff analysis) prove that
∅g = 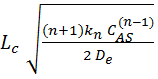 for nth order reaction.
for nth order reaction.
-rA=
kn CASn, ∅g = generalized Thiele
modulus for nth order reaction
2. A
catalytic elementary gas reaction of 2A → B +C was investigated for diffusion
effect using a differential flow reactor under the following conditions.
Amount
of catalyst = 50 grams
Spherical
catalyst with 0.6 cm diameter
Density
of catalyst = 1.6 grams/cm3
De
= 1.2x10-4 cm2/sec
Feed:
Pure A, P=3 atm, T=350°K, vo = 14 cm3/sec,
The
concentration of B (CB) coming out of the differential reactor was
measured to be 3.75x10-6 mols/cm3.
(a)
Calculate (-rA)obs based on the catalyst volume.
(b)
Using one-point method, estimate the effectiveness factor and the rate
constant.
Above
information is to be used to design a PBR
to carry out the following reaction. Feed conditions are the same as above.
Reactor is isothermal.
Additional
information: FAO
= 72 mols/hour
Required
conversion = 0.9
α
= 1.65x10-5 g-1
(c)
Calculate the required amount of catalyst. Plot conversion, y, and η vs. Vcatalyst.
Pay
attention to the units in concentration and time: cm3 vs. L, hour
vs. s.
Assignment No. 25
Due: 04/15, Fri
Problem
12-16 (a), (b)
Notes:
Keep units consistent: all volume units in cm3, time units in
seconds. Use linear regression.
The
reactor in this problem is a catalytic CSTR with W = 160 g.
Assignment No. 26
Due: 04/20, Wed
Derive
the RTD function for two CSTRs connected in series:
V1
= 10 liters, V2 = 20 liters, v0 = 10 liters/ minute.
Make
a plot of the RTD.
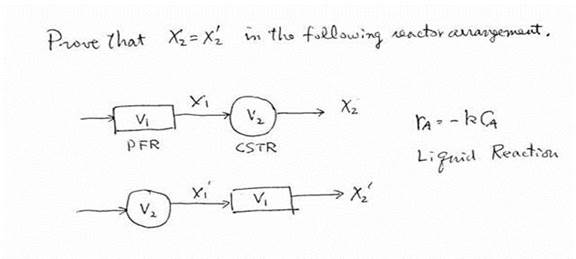
Prove
that ![]() .
.
Assignment No. 27
Due: 04/22, Fri
Using
Polymath, plot RTD function, and calculate τ and σϴ2 for Assignment 26. Repeat for
V1 = V2 = 15.
Assignment No. 28
Due: 04/25, Mon
1. From a tracer test of a
continuous reactor, the following data are obtained.
|
ti |
0 |
1 |
2 |
3 |
4 |
5 |
6 |
8 |
10 |
12 |
|
Ci |
0 |
1.2 |
4.0 |
3.5 |
3.0 |
2.6 |
2.0 |
1.0 |
0.4 |
0.0 |
(a) Using
Excel, estimate τ, sq2,
and conversion.
Additional data:
2nd
order reaction, k = 0.6 L/mol.minute, CA0
= 1.0 mol/L.
(b)
Compare the conversion with those of
PFR and CSTR.
2. Problem
13-19, (a)-(h), (m), (o), (p). (20
points)
Use Polymath.
Assignment No. 29
Due: 04/27, Wed
Problem 14-13, (d)-(g) (20 points)
Additional
notes:
For the sake of uniform answer, take σ2
= 75.4 and τ
= 9.93 from the previous HW 13-19.
Note
that τ1
= τ2 = τ/2
(d):
Ignore open system. Solve by polymath and also by Eq. 14-27, p971
(g):
Solve by mole balance using Polymath.