Antimicrobial Acrylic Fiber
INTC 2005 Proceedings
Jaewoong Lee1, R. M. Broughton1, Unchin Cho1, S. D. Worley2, J. Liang2, T-S. Huang3
1Department of
Textile Engineering,
2Department of
Chemistry,
3Department of
Nutrition and Food Science,
Abstract
Low molecular weight poly(styrenehydantoin) (PSH) was prepared from polystyrene polymer. PSH was dissolved in dimethylacetamide (DMAc) after which poly(acrylonitrile) (PAN) was added to the solution. Fibers were extruded by dry-jet wet spinning. The mechanical properties of the PSH/PAN blended fibers are presented. Biocidal efficacy and chloramine stability data are also discussed.
Key Words: Antimicrobials, Biocidal Fibers and Polymers, Modified Poly(acrylonitrile)
Introduction
Inactivation of bacteria which cause odor as well as contagious diseases is a desirable goal. Numerous antimicrobial materials, disinfectants, etc. have been developed over to the years as mankind has moved toward this goal. While it is desirable that biocidal materials should be strong inactivating agents for microorganisms, they should not be harmful to other life or the environment.
Fibers and fabrics are among the materials that are closely associated with humans, and for which antimicrobial functionality would be useful. One can easily recognize the benefit of biocidal hospital clothing and furnishings as well as upholstery, carpets and bedding for the general public.
Work on halamines as biocidal agents has been ongoing at
Applications for antimicrobial properties on fabrics are diverse. Coating treatments with biocidal chemicals are often used to produce antimicrobial materials, particularly fibers and fabrics. Due to the relatively low durability of coatings and the degradation of some physical properties, alternative treatment procedures are needed.
Since chemical bonding is the strongest connecting force between molecules, it should be the best way for application of antimicrobial compounds to fibers. However, as certain biocidal polymers have no appropriate functional groups for chemical bonding, blending with fiber-forming polymeric materials to make antimicrobial fibers can be a useful alternative.
Poly(acylonitrile) (PAN) is one of the standard fiber-forming materials, and has been used as a wool substitute because it is easily made into a bulky yarn. Since PSH and PAN are both soluble in dimethylacetamide (normally used for the extrusion of PAN), the solution might provide a route to fibers containing both polymers. An acrylic fiber containing PSH (Figure 1) might be expected to allow formation of chloramines on the surface and therefore be useful as an antimicrobial fiber.
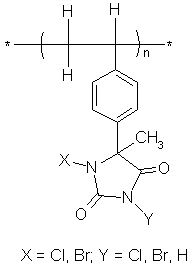
Figure 1 Structural formula of the halogenated poly(styrenehydantoin) compound
Experimental
Materials
PSH used was synthesized by an established procedure [5] in a molecular weight range of 800 - 5000. The PAN was a fiber forming acrylonitrile copolymer, and obtained from Solutia Inc. All solvents, unless otherwise stated, were of reagent grade, and used without further purification.
Preparation of PAN/PSH solution
Appropriate amounts of PSH were dissolved in 70mL of dimethylacetamide (DMAc), after which 7g of PAN was added to the DMAc/PSH to produce a solution of approximately 10% PAN. The ratios of PAN/PSH were varied from 100/0 to 100/12(by weight). The blended solution was stirred for 24 h at 70ºC. The solution was allowed to store for more than 2 h at 70ºC without stirring to remove air bubbles, after which it was poured into a piston wet spinning apparatus.
Wet spinning process
The piston pump was fitted with a single hole (1.25 mm diameter circular) spinneret. The set-up consisted of extruder, a coagulating bath (using tap water), a stepped godet with four levels and take-up winder. The blended solutions in the extruder cylinder were maintained 35ºC, and forced through the spinneret fitted with a 325 mesh wire screen filter inside. Extrusion conditions were as follows:
Process: dry-jet wet spinning,
Extrusion temperature: 35ºC
Throughput: 0.16~0.64 mL/min
Coagulation bath: tap water
Godet speed: 12.5~50 RPM
Drawing: 3 stage, steps 1, 2, 3, and 4
Draw ratio: 3.9
Take-up speed: 2.0 - 8.1 m/min
After wet spinning, the yarns were soaked in tap water at ambient temperature for 24 h to extract the solvent which was used for spinning.
Measurement of Physical Properties
Tensile properties were investigated with a Universal tensile tester at 22ºC and 65% humidity. The data were obtained by averages of 10 tests. The length between upper and lower jaw was 25.4mm, and crosshead speed was 20 mm/min.
Chlorination and Titration
The fibers were chlorinated using a commercial, 6% sodium hypochlorite solution (diluted to 10% of the commercial strength with distilled water) at pH 8 to produce biocidal materials. After soaking in the solution at ambient temperature for 30 min, and rinsing with distilled water, the samples were dried at 45ºC for 2 h to remove the unbonded chlorine.
An iodometric/thiosulfate titration procedure was used to analyze oxidative chlorine content. Through the following equation, [Cl+] % in the sample was calculated:
[Cl+] % = (V x N x 35.45) / (W x 2 x 10)
Where [Cl+] % is the wt% of oxidative chlorine on the sample, V the volume of the titrant, sodium thiosulfate solution (mL), N the normality of the titrant, W the gram weight of the sample(g).
Laundering Test
AATCC Test Method 61-1986 was used to investigate the stability of PSH and chlorine in/on the fibers after home laundering. A Launder-Ometer fitted with stainless cylinders (3 x 5 in.) including 150 mL of 0.2 % AATCC detergent solution and 50 stainless steel balls was rotated for 45 minutes at 42± 0.5 RPM and 49ºC. These conditions are estimated to be equivalent to 5 washing cycles in a home laundry. After detaching the cylinders, the fibers inside cylinder were rinsed with three 300-mL portions of distilled water, and air dried at ambient temperature.
Antimicrobial Test
After making the fibers into a crude nonwoven fabric, treated samples were challenged with Staphylococcus aureus (ATCC 6538) using a modified AATCC Test Method 100-1999. After applying bacteria suspensions in pH7 phosphate buffer solution to the samples, and covering with another swatch for 30 minutes contact time, the samples were quenched with 0.02 N sodium thiosulfate solution. Serial dilutions with pH7 phosphate buffer were plated on nutrient agar. Incubation for 48 h was followed by counting to determine the presence or absence of viable bacteria.
Results and Discussion
Properties and Chlorine Content of the
Fibers
The tensile properties of samples were measured at 65% humidity and 22oC after conditioning the samples for 24 h. The results are shown in Table 1. The data indicate that increasing the PSH content of the fiber increases the chlorine content after chlorination; however, as the PSH content increased, tenacity was decreased; however extrusion condition were not optimized for fiber properties. It is presumed the presence of the PSH may lower packing density of PAN resulting in a decreased tenacity. .
Table 1 Mechanical properties and chlorine content of the acrylics(0.2mL/min of extrusion velocity and 50RPM of a take-up speed)
|
Ratioa (PAN/PSH) |
100/0 |
100/4 |
100/8 |
100/12 |
|
[Cl+] % |
0 |
0.01 |
0.05 |
0.07 |
|
Denier (g/9000m) |
21.2 ±4.1 |
20.1 ±2.7 |
27.9 ±5.8 |
29.2 ±5.3 |
|
Tenacity (g/den) |
1.50 ±0.16 |
1.33 ±0.11 |
0.99 ±0.05 |
0.92 ±0.16 |
|
Strain at break (%) |
55.3 ±15.8 |
54.5 ±10.6 |
12.3 ±16.5 |
15.6 ±11.5 |
a PAN/PSH by weight
As denier decreased, the blended acrylic released (absorbed) more chlorine. The results are indicated in Table 2. It is suggested that poly(acrylonitrile) has limited moisture absorption due to inherent hydrophobicity, and as a result, only PSH on the surface of the fiber is active in absorbing chlorine and inactivating bacteria. Due to the theoretical Cl+ [%] of PSH is 24.87, the theoretical Cl+ [%] of PAN/PSH(100/12) should be 2.66. We have chlorinated 4.13% of the available PSH sites when denier was 21. Since thinner fibers have more surface area, one would expect that thinner fibers would have greater chlorine retention and antibacterial effect at lower PSH content. The effect of surface area on absorbed chlorine is shown in Figure 2.
Table 2
Variation of chlorine content of PAN/PSH(100/12) fibers with various
deniers (Take-up speed: 12.5RPM)
|
Extrusion velocity (mL/min) |
Denier (g/9000m) |
Surface area (m2/g) |
[Cl+] % |
Atoms/cm2 |
|
0.64 |
398 |
1.57 x 10-2 |
0.02 |
2.16 x 1016 |
|
0.32 |
179 |
2.34 x 10-2 |
0.04 |
2.90 x 1016 |
|
0.16 |
85 |
3.39 x 10-2 |
0.07 |
3.51 x 1016 |
|
0.16a |
21b |
6.86 x 10-2 |
0.11 |
2.72 x 1016 |
|
N/A |
1 |
31.10 x 10-2 |
0.84b |
4.59 x 1016 |
a Take-up speed: 50RPM
b Predicted value
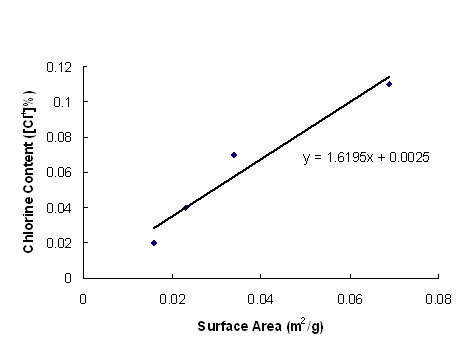
Figure 2 Chlorine content of PAN/PSH(100/12) fibers vs fiber specific surface area
Using the graph in
Figure 2 and extrapolating to a surface area corresponding to a denier of 1, we
would expect a Cl+ content of 0.84 % which corresponds to 31.5% of
theoretical chlorine absorption (if all nitrogen absorption sites were used).
Durability, Rechargeability and
Antimicrobial Efficacies
The relative durability of chlorine to laundering and rechargeability afterwards (with Cl) were evaluated; the results appear in Table 3. All of blended acrylics retained little of their antimicrobial properties after 50 washes; however, after recharging, the fibers with household bleach, they regained almost all of their original chlorine content.
Table 3 Durability and rechargeability of the acrylics (0.16mL/min of extrusion velocity and 50RPM of a take-up speed)
unit: [Cl+] %
|
Ratioa (PAN/PSH) |
100/0 |
100/4 |
100/8 |
100/12 |
|
Chlorinated sample |
0 |
0.03 |
0.07 |
0.11 |
|
Washing 50 cycles |
0 |
0.01 |
0.01 |
0.05 |
|
Recharge after washing 50 cycles |
0 |
0.04 |
0.07 |
0.10 |
a PAN/PSH by weight
The fibers were formed into a nonwoven matt which upon chlorination with 10 % household bleach became antimicrobial. The chlorinated nonwoven (0.2g) was separated into two pieces for the swatch test. The results are shown in Table 4. From the data, most of chlorinated acrylic nonwovens caused complete inactivation of S. aureus in 30 min.
Table 4 Antimicrobial efficacy of PAN/PSH blended fiber against S. aureus
|
Samples |
Contact time (min) |
Bacterial reduction |
||
|
(%) |
Log Reduction |
|||
|
Unchlorinated fibers |
PAN/PSH(100/0) |
30 |
49 |
0.2 |
|
PAN/PSH(100/4) |
30 |
32 |
0.1 |
|
|
PAN/PSH(100/8) |
30 |
88 |
0.9 |
|
|
PAN/PSH(100/12) |
30 |
99.5 |
2.2 |
|
|
Chlorinated fibers |
PAN/PSH(100/0) |
30 |
94 |
1.2 |
|
PAN/PSH(100/4) |
30 |
99.9 |
3.3 |
|
|
PAN/PSH(100/8) |
30 |
100 |
6.5 |
|
|
PAN/PSH(100/12) |
30 |
100 |
6.5 |
|
Each sample was inoculated with 25 µl of bacterial
suspension at 3 x 106
cfu/ml.
Conclusions
Modified acrylic fibers (a blend of PAN/PSH) were produced by dry-jet wet spinning. Strength properties and antibacterial effectiveness of the fibers were investigated. Strength properties were somewhat decreased as more PSH was added during extrusion. As the PSH content increased, the absorbed chlorine and antibacterial effectiveness also increased. Since poly(acrylonitrile) is hydrophobic (has limited moisture absorption), it might be presumed that just PSH on the surface of the fibers is active in absorbing chlorine and inactivating bacteria. The fact that only a fraction of the theoretical chlorine absorption was observed and the fact that absorbed chlorine is shown to increase with increasing surface area of the fibers, supports this theory. The treatment appears stable to repeated laundering.
Acknowlegdements
This research was supported by the US Air Force and by the
References
[1] S. D. Worley and D. E. Williams, “ Halamine Water Disinfectants”, Crit. Rev. Environ. Control 18, 133 (1988)
[2] S. D. Worley and Sun, G., “Biocidal Polymers”, Trends in Poly. Sci., 4, 364 (1996)
[3] S. D. Worley and H. D. Burkett, “The Stability in Water of a New Chloramine disinfectant as a Function of pH, Temperature, and Water Quality”, Water Res. Bull., 20, 365 (1984)
[4] S. D. Worley, W. B. Wheatley, H. H. Kohl, H. D. Burkett, J. H. Faison, J. A. VanHoose, and N. Bodor, “A Novel Bactercidal Agent for Treatment of Water”, Water Chlorination: Environmental Impact and Health Effects, Vol.4, ed. R. L. Jolley, 1982, pp. 1105-1113
[5]
Sun, G.; Wheatley, W. B.; Worley, S. D., “A New Cyclic N-Halamine Biocidal Polymer”,
Ind.
[6] M. W. Eknoian, S. D. Worley, J. Bickert, J. F. Williams, “Novel Antimicrobial N-Halamine Polymer Coatings Generated by Emulsion Polymerization”, Polymer, 40, 1367 (1999)
[7] S. D. Worley, Y. Chen, J. Wang, R. Wu, J. F. Williams, J. Chen, and Y. Li, “Novel N-Halamine Siloxane Monomers and Polymers for Preparing Biocidal Coatings”, International Meeting on Hygienic Coatings, Paint Research Association, Orlando, FL, Jan. 2004.
[8] M. W. Eknoian and S. D. Worley, “New N-Halamine Biocidal Polymers”, J. Bioact. Compat. Polym., 13, 303 (1998)
[9] J. Liang, R. Wu, T. S. Huang, “Polymerization of a Hydantoinylsiloxane on Particles of Silicon Dioxide to Produce a Biocidal Sand”, J. Appl. Polym. Sci., in Press
[10] T. J. Elder, S. D. Worley, “The Application of Molecular Orbital Calculations to Wood Chemistry. IV. The Formation of Methylol Derivative”, J. Wood Chem. and Tech., 6, 505 (1986)
[11] S. D. Worley, J. Liang, R, Wu, J-W. Wang, K. Barnes, U. Cho, J. Lee, R. M. Broughton, and T. S. Huang, “N-Halamine Biocidal Coatings”, Soc. Ind. Microbiol, Biofilms 2005 Symp., Arlington, VA, Apr. 2005.
[12] S. D. Worley, Y. Chen, J. Wang, R. Wu, J. F. Williams, J. Chen, and Y. Li, “Novel N-Halamine Siloxane Monomers and Polymers for Preparing Biocidal Coatings”, International Meeting on Hygienic Coatings, Paint Research Association, Orlando, FL, Jan. 2004.