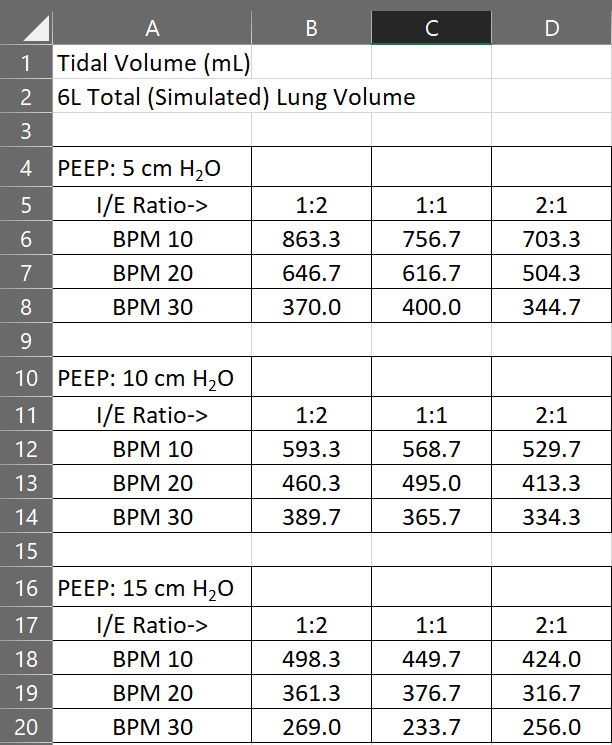
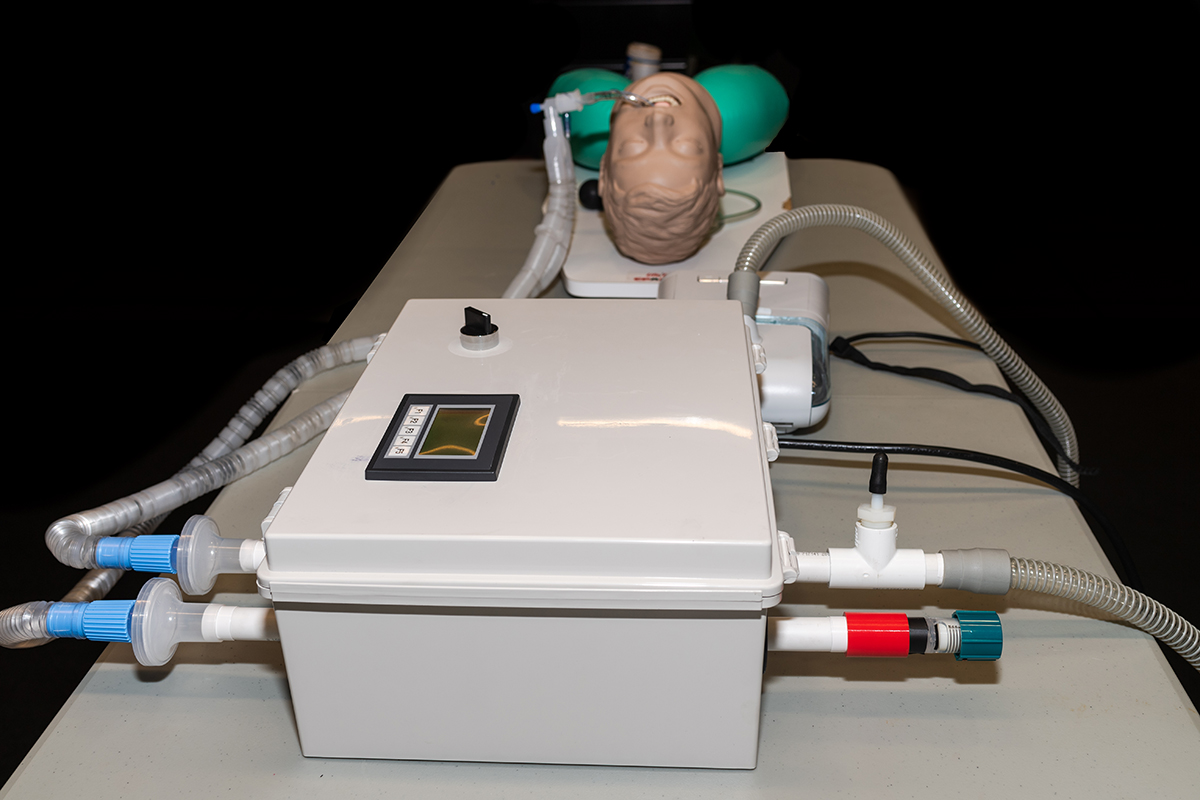
A team of Auburn University engineering professors, students and alumni have successfully re-purposed a standard CPAP machine into a functional emergency ventilator for health care providers potentially coping with ventilator shortages during the Coronavirus Disease 2019 (COVID-19) crisis. The team’s research goal was to develop a robust and reliable option for an emergency ventilator that could be assembled using readily available commercial off-the-shelf components combined with a home CPAP machine.
Currently on its third iteration after more than one week of bench tests, the Auburn RE-INVENT Project was launched not just to develop a low-cost solution, but rather to focus primarily on developing a device that checked every box for reliably ventilating a patient for an extended period during this public health emergency. To this end, the team worked closely with Dr. Glenn Woods, a local anesthesiologist with first-hand experience utilizing ventilators for medical care.
Built with approximately $700 worth of components, the RE-INVENT is an accessory designed to pair with, and modify, a common household CPAP machine.
The prototype was developed in just two days (March 20-22) by Tom Burch and Michael Zabala, faculty in the Samuel Ginn College of Engineering's Department of Mechanical Engineering, and Hayden Burch, a sophomore in mechanical engineering. Between March 22 and March 30, additional engineering faculty and alumni joined the team to refine the mechanical design, control system, user interface, and alarm.
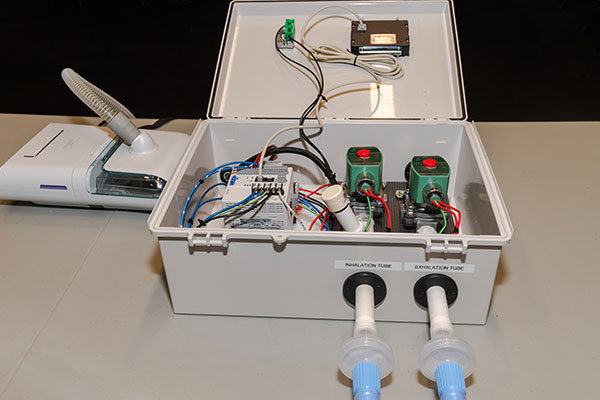
The emergency ventilator system is composed of the common CPAP machine, the RE-INVENT valve assembly, and the standard tubing and tracheal tube that is used in current ventilators to deliver air to the patient. RE-INVENT contains an inhalation valve and an exhalation valve, both rated for constant operation on a 100% duty cycle, and a PLC-based control system with user interface and alarms.
The CPAP machine maintains a positive pressure of air into RE-INVENT at all times. Oxygen may be bled into RE-INVENT upstream from the inhalation valve. As the inhalation valve opens, warmed and moisturized air from the CPAP machine enters through the viral filter and into the inhalation hose to the patient. During inhalation, the exhalation valve remains closed. As the inhalation valve closes, the exhalation valve opens and allows air to exit the lungs down the 22 mm exhalation tube, through the viral filter, through the exhalation valve, through a PEEP valve and finally out into the atmosphere. Exhalation occurs naturally due to the return to shape of the patient’s lungs and chest.
RE-INVENT can provide a range of inspiration to expiration ratios between 1:3, and 3:1 on increments of 0.1. It can also provide a range of 10 to 30 breaths per minute.
The air pressure is set directly on the CPAP machine (normally between 5 and 25 cm H2O). The inspiration/expiration ratio and breath rate (breaths per minute) are set by the attending health care professional using the digital interface on RE-INVENT. The patient will have their CO2 monitored and their breathing rate monitored via EKG by health care professionals. If either of these patient parameters surpass a set threshold, alarms will alert nearby healthcare professionals.
The tracheal tube and the bacterial/viral filters at the ends of the standard 22 mm hoses shown in the above demonstration video are the same that would be used on a patient treated with a standard ventilator. Both of the filter housings on the 22 mm hoses attach directly to the RE-INVENT assembly. The bacterial/viral filters are identical to what is used on current ventilators and serve to prevent aerosolization of the virus.
The RE-INVENT team continues to aggressively conduct research and development to refine and validate the efficacy of the design. While this testing and development continues, the RE-INVENT concept appears to be an emergency option for consideration by health care providers when traditional ventilator options are not available for the patient. On March 22, 2020, the FDA released a letter to health care providers titled "Ventilator Supply Mitigation Strategies." We encourage health care providers to review this guidance from the FDA as they consider whether the RE-INVENT may be an option for them as an emergency ventilator.
FDA GUIDANCE
On March 22, 2020, the U.S. Food and Drug Administration (FDA) issued immediate guidance outlining a policy intended to help increase availability of ventilators and their accessories as well as other respiratory devices during the COVID-19 pandemic. For details, see the letter to health care providers titled "Ventilator Supply Mitigation Strategies."
- Letter to Health Care Providers: Ventilation Supply Mitigation Strategies
- Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency
- Ventilator Emergency Use Authorization Letter
Large Animal Testing
On April 3, 2020, large animal testing was conducted with the RE-INVENT system at the JT Vaughan Large Animal Teaching Hospital within the Auburn University College of Veterinary Medicine. The test protocol was reviewed and approved by the Auburn University Institutional Animal Care and Use Committee (IACUC).
The animal testing investigators consisted of Dr. Stuart Clark-Price, DVM, MS, DACVIM, DACVAA, CVA, Associate Professor Anesthesia/Pain Management, Department of Clinical Sciences in the AU College of Veterinary Medicine; Dr. Terry Brandebourg, Associate Professor, Department of Animal Sciences, in the AU College of Agriculture; Dr. Michael Zabala, Assistant Professor, and Dr. Thomas Burch, Senior Lecturer, both in the Department of Mechanical Engineering, AU Samuel Ginn College of Engineering.
For the test, a healthy male goat weighing 89 kg (196.2 lbs) was anesthetized, intubated, and paralyzed with similar medications that are used in human medicine and then ventilated with the RE-INVENT system paired with a Philips Respironics DreamStation CPAP machine for approximately two hours. The CPAP was set to a pressure of 20 cm H2O for the duration of testing. Multiple conditions of testing were performed by altering parameters including breath rate (BPM), inspiration to expiration ratio, oxygen supply, and PEEP (positive end-expiratory pressure). Observing via video conferencing were other team members including Dr. Glenn Woods, MD, Anesthesiology and Pain Management. Oxygen saturation, EtCO2, Inspiratory Pressures and Tidal Volume all remained within acceptable parameters using PEEP ≤ 10 cm H2O and a respiratory rate between 10-15 breaths per minute. Arterial blood gases were measured at various timepoints throughout the testing period and remained within normal reference ranges. After the successful testing was complete, the goat was allowed to gently regain consciousness and verified to be healthy and fit.
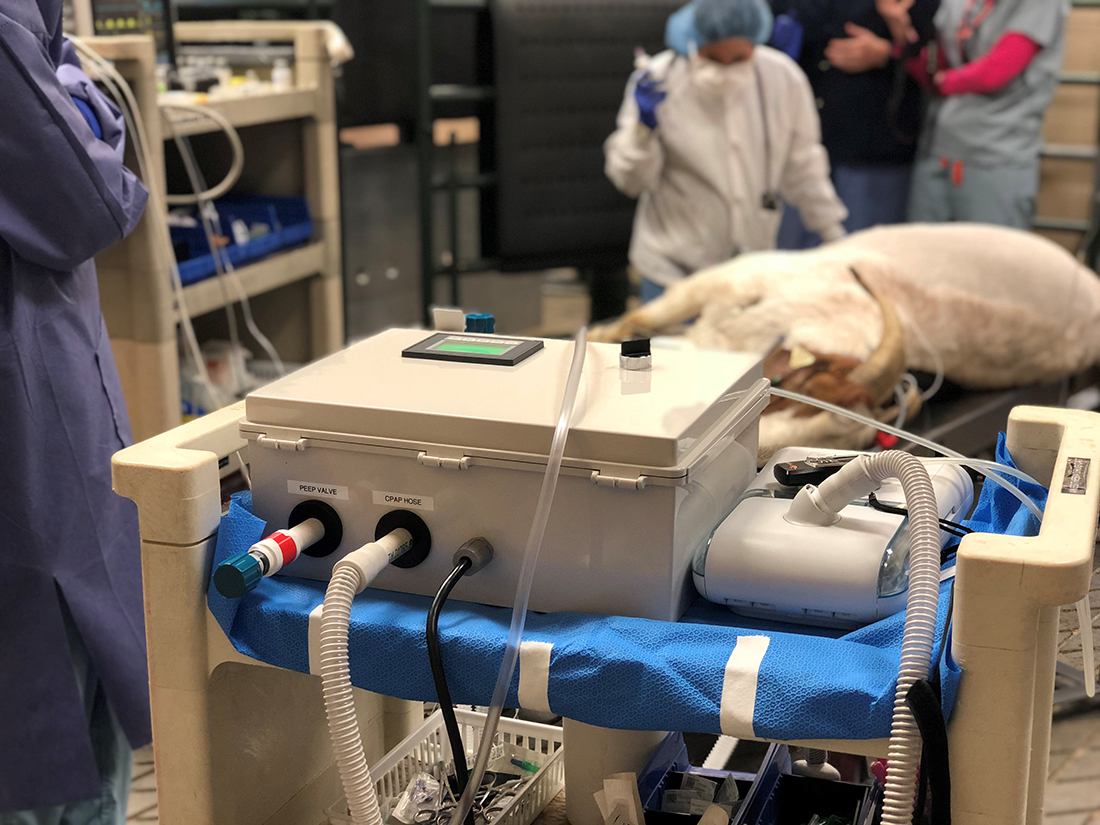
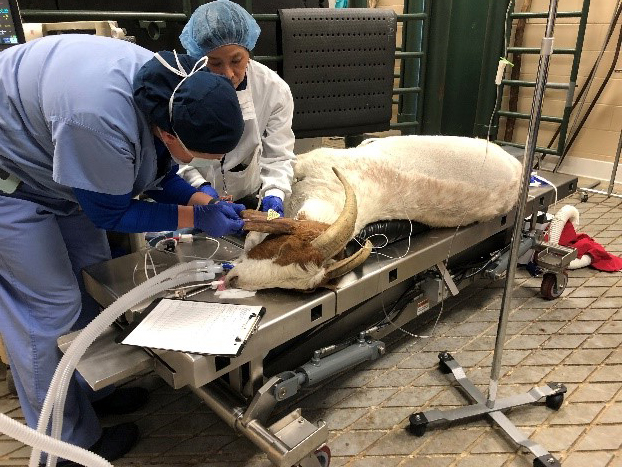
Figure 1. 200 lb. goat ventilated with RE-INVENT and Philips Respironics DreamStation CPAP.
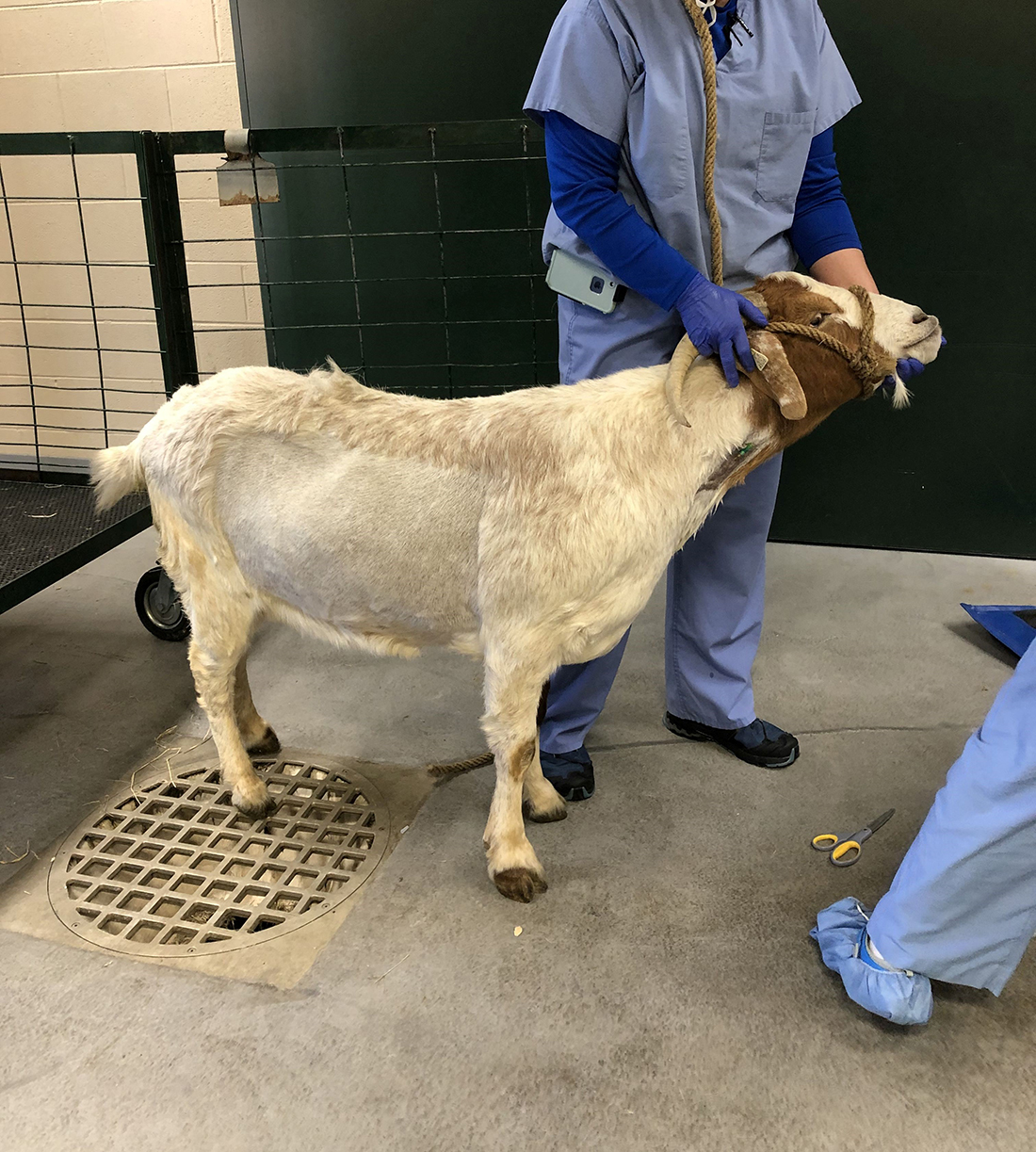

Figure 2. Before and after testing.
Tidal Volume Testing
A TSI® Certifier® High Flow Module was used to measure tidal volume with a pair of 3 L (each) simulated lungs and an ACLS manikin under various conditions of PEEP, breaths per minute (BPM), and insipiration/expiration ratio (I/E). The results are tabulated below.
Early Benchmark Testing
The first round of benchtop testing utilized a simulated lung. This test demonstrated the capability of the RE-INVENT and the CPAP machine to produce consistent results under different inspiration times and breath rates and different amounts of PEEP (Figure 1).

Figure 1. 1st round of benchtop testing with Gen 1 design on a simulated lung.
The second round of benchtop testing occurred in a Respiratory Therapy Lab (Figure 2). This round of testing included use of a bedside spirometer. We were able to achieve about 500 ml of tidal volume for only one simulated lung. Results were considered successful under different inspiration times and breath rates and different amounts of PEEP.


Figure 2. 2nd Round of benchtop testing with Gen 1 on a simulated lung.
The third round of testing was conducted on a QuickLung test lung. This test occurred under various inspiration times and breath rates and different amounts of PEEP and was considered successful.



Figure 3. 3rd round of testing with Gen 2 on the QuickLung
TEAM
- Hardware Design/Fluid Mechanics: Tom Burch, Ph.D, P.E. — senior lecturer, mechanical engineering (B.S. ’79 M.S. ’82 Ph.D ’90)
- Biomechanics: Michael Zabala, Ph.D — assistant professor, mechanical engineering (B.S. M.E. ’07)
- Computer Aided Modeling and Mechanical Design: Joe Ragan, Ph.D — lecturer, mechanical engineering
- Control Systems: Mike Hamilton, Ph.D — professor, electrical and computer engineering (B.S. E.E ’00)
- Hardware Design: Ryan Hill, M.S. — research mechanical engineer, IS4S, (B.S. M.E. ’08, M.S. M.E. ’11)
- Fluid Mechanics: Justin Harrison, M.S. — principal research engineer, University of Alabama Huntsville (B.S. M.E. ’07, M.S. M.E. ’14)
- Instrumentation and Controls: Jim Chapman — instrumentation and controls engineer, Sanders Lead Company (M.E. ’98)
- Hayden Burch, mechanical engineering student
- Rob Engle, MBA | M.S.M.E.
- Glenn Woods, M.D. — anesthesiology and pain management